|
|
 |
 |
| Home » International Products » EU - British |
 |
EU British
Founded in 1996 in San Diego, California, MacroPore Biosurgery has expanded its biomaterials platform technologies to develop and provide even greater medical solutions for the biosurgery market. Biomaterials technology includes bioresorbable polymer implants for use in spine, neurosurgical, and other musculoskeletal applications, and bioresorbable thin films for soft tissue applications.
MacroPore Biosurgery completed its initial public offering in Germany on August 8, 2000, and its common stock is traded on the Frankfurt Stock Exchange under the symbol XMP.
Reconstructive Implants and Bioresorbable Materials
Historically, reconstructive implants have been made of permanent materials such as metal or ceramic. Permanent or nonresorbable implants remain in the body after healing takes place. In select cases however, the implant is performing a temporary task, and is not required after healing is completed. Bioresorbable implants are made from molecules similar to those in the human body and resorb after the tissue is healed.
Bioresorbable Polymer Implants
Bioresorbable polymer implants are made from essentially the same lactic acid molecular building blocks that occur naturally in the human body. (Lactic acid is produced in the muscles during strenuous activity.) Long molecular polymer chains are created by combining lactic acid derivatives known as lactides. The resulting polymers are generally referred to as polylactides or PLa. MacroPore Biosurgery implants, used for hard tissue (bone) and soft tissue applications, are made from Polylactide: a copolymer of 70:30 Poly(L-lactide-co-D,L-lactide).
Material Biochemistry
The copolymer maintains its strength during the healing process, and through hydrolysis slowly breaks down into lactic acid molecules. The resorption process occurs in two phases: (1) H2O (water) penetrates the implant, reacts with the polymer and breaks the polymer chains (hydrolysis); (2) hydrolysis converts the long chains into shorter chains until the polymer fragments into single lactic acid molecules. Lactic acid molecules are then metabolized by the liver into CO2 (carbon dioxide) and H2O and released through the lungs.
Bioresorbable polymers provide relatively high strength and predictable resorption rates for specific hard or soft tissue applications. Different resorption rates are beneficial for various surgical applications. For example, rapidly healing tissue (pediatric) may require an implant with a faster resorption rate than an implant designed for adult tissue that may experience slower or impaired healing. The resorption rates are controlled through material selection and fabrication methods.
Bioresorbable Polymer Implant Advantages
- Bioresorbable, biocompatible, and "biofriendly."
- Application versatility designed for hard tissue (bone) and soft tissues.
- Predictable resorption rate.
- Avoids risk of disease transmission associated with human or animal materials.
- Does not obscure radiographs or MRI/CT scans.
- Strength appropriate for specific applications.
- Reduced risk of long-term failure requiring re-operation.
- Reduced risk of stress shielding or weakening the healing bone (rigid metal implants may weaken bone over time).
Bioresorbable Products
Spine and Orthopedics - building on the initial biomaterials platform technology for hard tissue (bone) applications, MacroPore Biosurgery focuses on bioresorbable polymer implants to maintain the relative position of weak bony tissue such a bone grafts or bone graft substitutes, as well as other musculoskeletal reconstructive applications.

HYDROSORB" Telamon bioresorbable lumbar spine cages used for spine graft containment.
In January 2003, MacroPore Biosurgery received CE Mark approval to market HYDROSORB" Telamon resorbable lumbar spine cages in Europe. These implants are used when vertebral disks are damaged after trauma, for disease or age related degeneration, and when a spinal fusion procedure is necessary.
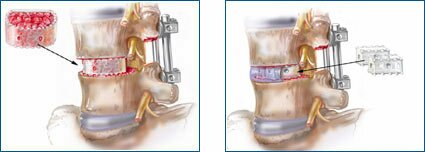
Illustrations depict HYDROSORB" bioresorbable implants used to promote spinal fusion by maintaining the relative position of bone grafting materials and to assist in maintaining the space between vertebral bodies in conjunction with rigid fixation. Once fusion occurs the bioresorbable implants are resorbed by the body.
The spine and orthopedics bioresorbable products are manufactured by MacroPore Biosurgery and are co-developed, marketed and distributed throughout Europe by Medtronic Sofamor Danek.
Bioresorbable Thin Films expansion of the biomaterials platform technology includes bioresorbable thin films for soft tissue support and repair, and the control of postsurgical adhesions. Bioresorbable thin films have clinical applications across multiple surgical specialties.

Bioresorbable adhesion barrier films are used for soft tissue applications.
HYDROSORB" Shield has received the CE Mark for temporary wound support, to aid in reoperation procedures by promoting the formation of a surgical dissection plane, and to control the formation of adhesions in specific spinal applications. HYDROSORB" Shield products are manufactured by MacroPore Biosurgery and are co-developed, marketed and distributed throughout Europe by Medtronic Sofamor Danek.
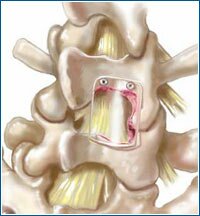
Illustration depicts bioresorbable film application
MacroPore OS" is a US trademark of MacroPore Biosurgery, Inc.
HYDROSORB" is a trademark of Medtronic Sofamor Danek.

|
 |
|
 |



